Reveal new insights about medical devices
Frequently Asked Questions
The FDA's 510(k) program provides clearance for 99% of U.S. human medical devices. The 510(k) clearance process allows medical device manufacturers to market their device as long as it is "substantially equivalent" to a legally marketed device.
Read more on fda.gov.
No, this website is not affiliated with the FDA. All data on 510k.fyi is derived from the official FDA 510(k) database independently. This website is meant to be used as reference, but all information should be verified with the official FDA database.
This website can be used free of charge. All data from the FDA is public domain, and the code for this website is licensed under an open source license. Source code.
The 510k program spans a wide array of device types: from hip replacements to latex gloves. The FDA maintains a list of product types at accessdata.fda.gov.
Features
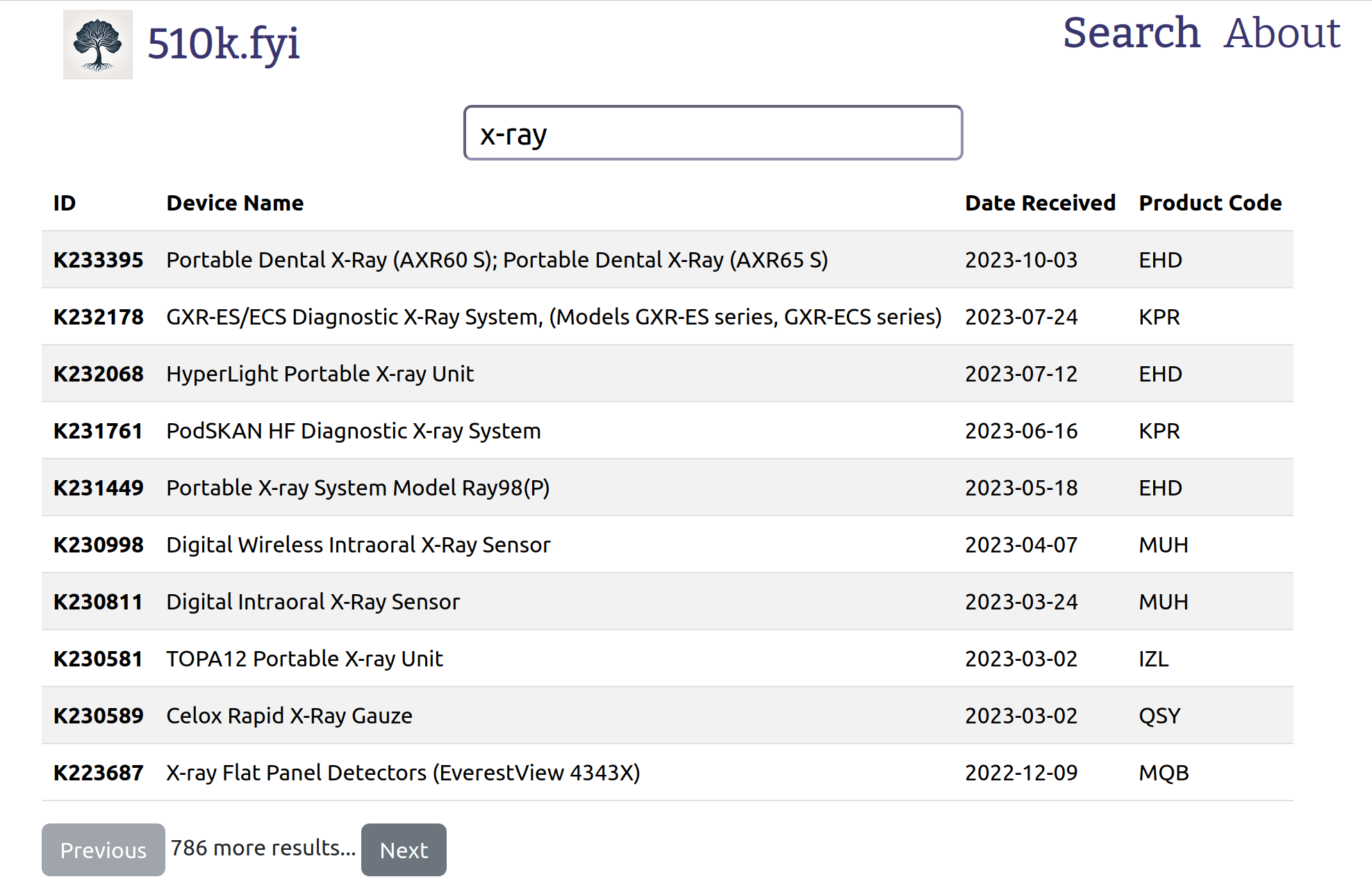
Search for devices
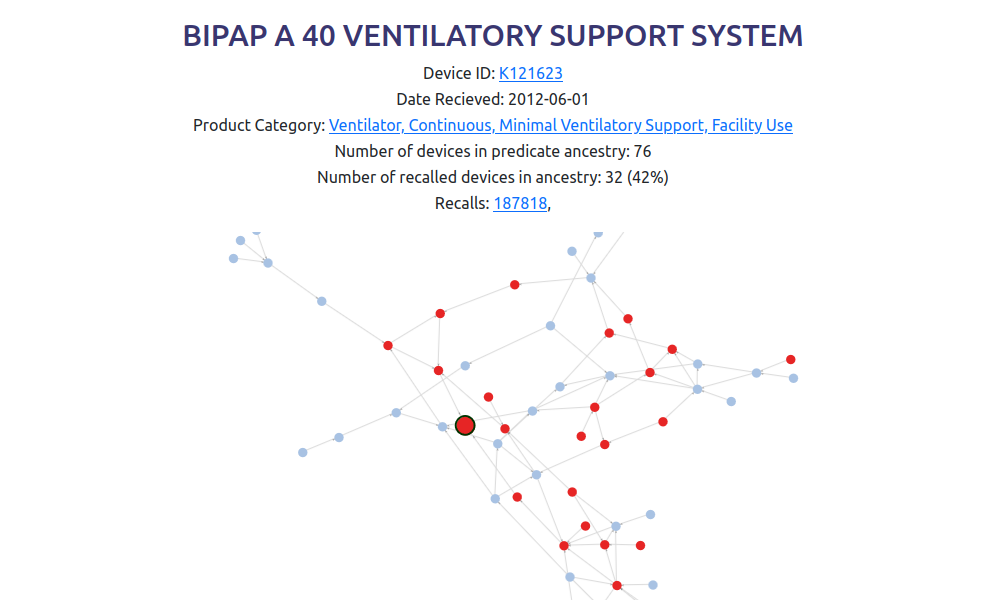
Find device predicate ancestry